What is the formula of Cobalt Sulfide?
The chemical formula is CoS because the Cobalt has (II ) 2 positive charged ions and the Sulfur also 2. That is why only 1 cobalt II can bind to 1 Sulfur.
Cobalt Sulfide
What is Cobalt diSulfide or CoS2?

Cobalt disulfide exists because there exist also Sulfur that is 1-( I -) it has only one negative charge. Sulfur can exist under different forms and this often confuses chemical students.
What is the difference between Cobalt Sulfide and Cobalt disulfide (CoS2)?
With Cobalt sulfide designs a whole range of atomic different structures with a formula CoxSy. They are usually black and used for titration.

Cobalt powder
We can distinguish some frequent occurring molecular structures like Co(II)S, (Co2S2), Co(I)S2, Co3S4, and Co9S8.
In Co9S8 we see Co can have in the same molecule different positive + ions and S has 2 or 3 negative anions
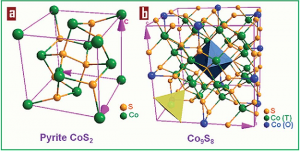
Pyrite
Why are there so many Cobalt molecular combinations?
The valence or charge of Cobalt can be from -1 to +4
Co3S4 means Co(IV)3S(III)4
Sulfer can only occur with 2 or 3 negative valence Charges
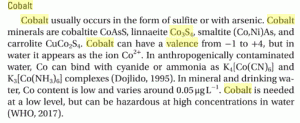
Cobalt
 Cobalt SD
Cobalt SD
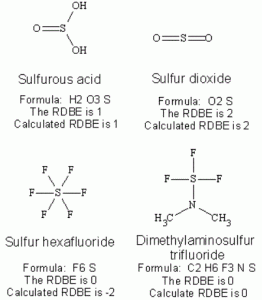
sulfur
Cobalt Sulfide Coloration
Pyrite is a very popular Cobalt sulfide.
Binary cobalt sulfide minerals include the cattierite (CoS2) and linnaeite (Co3S4).
CoS2 is isostructural with iron pyrite, featuring disulfide groups, i.e. Co2+S22−.
Co9S8 is quiet rare cobaltpentlandite (the Co analogue of pentlandite).
Cobalt sulfide minerals are converted to cobalt via roasting and extraction into aqueous acid. In some processes, cobalt salts are purified by precipitation when aqueous solutions of cobalt(II) ions are treated with hydrogen sulfide.
Whay is there also Cobalt Trisulfide?
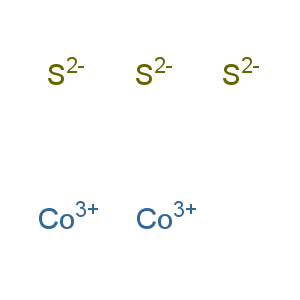
Cobalt trisulfide
- Iupac Name:cobalt(3+);trisulfide
- CAS No .: 12013-10-4
- EINECS(EC#): 215-273-3
- Molecular Weight:214.046
- Molecular Formula:Co2S3 (isomer)
[Linking template=“default“ type=“products“ search=“Cobalt Ii Sulfide Formula“ header=“2″ limit=“70″ start=“1″ showCatalogNumber=“true“ showSize=“true“ showSupplier=“true“ showPrice=“true“ showDescription=“true“ showAdditionalInformation=“true“ showImage=“true“ showSchemaMarkup=“true“ imageWidth=““ imageHeight=““]